Metabolic Disease
Diabetes
Diabetes is a complex metabolic disorder characterized by hyperglycemia (high blood glucose), which results when the body produces an inadequate amount of insulin – a hormone that allows blood glucose to enter cells – or when the body is resistant to the activity of insulin. Type 1 diabetes is an autoimmune disease in which insulin-producing cells of the pancreas are destroyed. In type 2 diabetes, certain cells throughout the body have a decreased response to insulin – also known as insulin resistance. Both types of diabetes can lead to long-term complications from persistent hyperglycemia, including blindness, cardiovascular disease, and kidney failure.
Of the approximately 90 million people in the United States and European Union who have diabetes, 17 million use exogenous insulin treatment – such as insulin injections and insulin pumps – to manage their disease.
Current treatments for type 1 diabetes require burdensome, chronic use of insulin pumps or multiple daily insulin injections, as well as constant treatment modification based on dietary carbohydrate intake and blood glucose monitoring. Despite the availability of new technologies for glucose monitoring and insulin administration, 70 to 80 percent of people with type 1 diabetes still have blood glucose levels that exceed their targets, increasing the risk of long-term complications.
Kriya is developing KRIYA‑839, a one-time gene therapy for diabetes expressing insulin and glucokinase, delivered intramuscularly with the objective of driving durable glycemic control and reducing or eliminating the need for exogenous insulin. Kriya is initially evaluating its gene therapy in type 1 diabetes.
Kriya has designed KRIYA‑839 for diabetes with the following potential goals in mind:
- Robust glycemic control. Improving time-in-range and lowering HbA1c to reduce the risk of long-term diabetic complications
- Insulin independence. Enabling patients to achieve insulin independence while maintaining robust glycemic control, offering the possibility of a functional cure
- Minimized treatment burden. Substantially reducing or eliminating the need for multiple daily injections of insulin with a one-time treatment
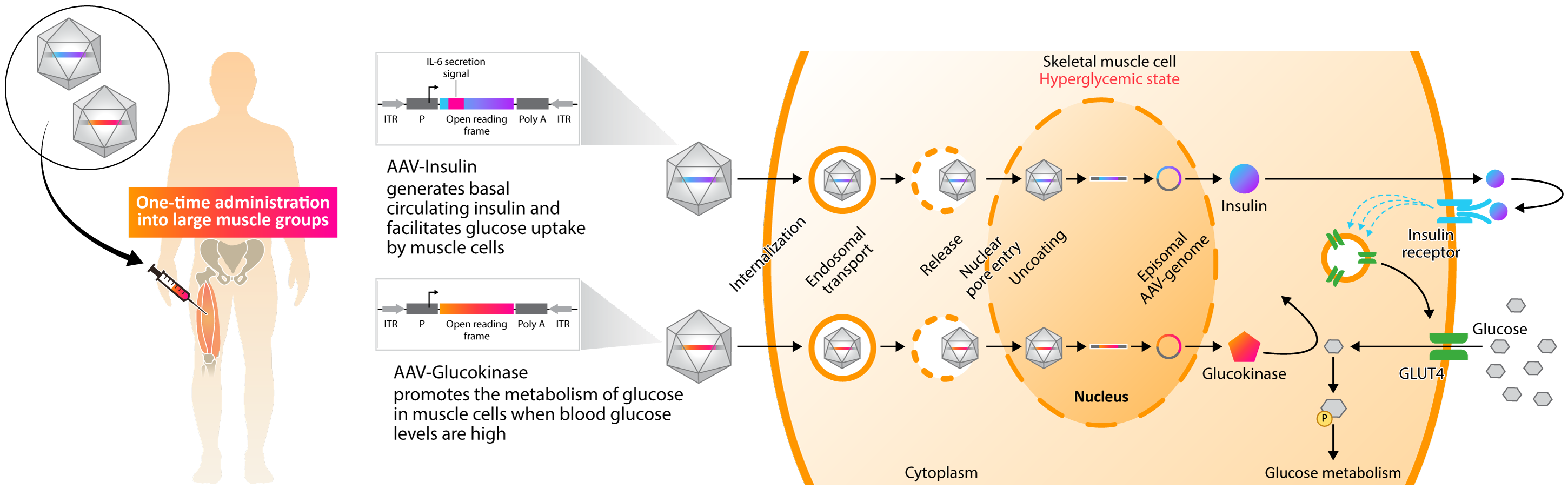
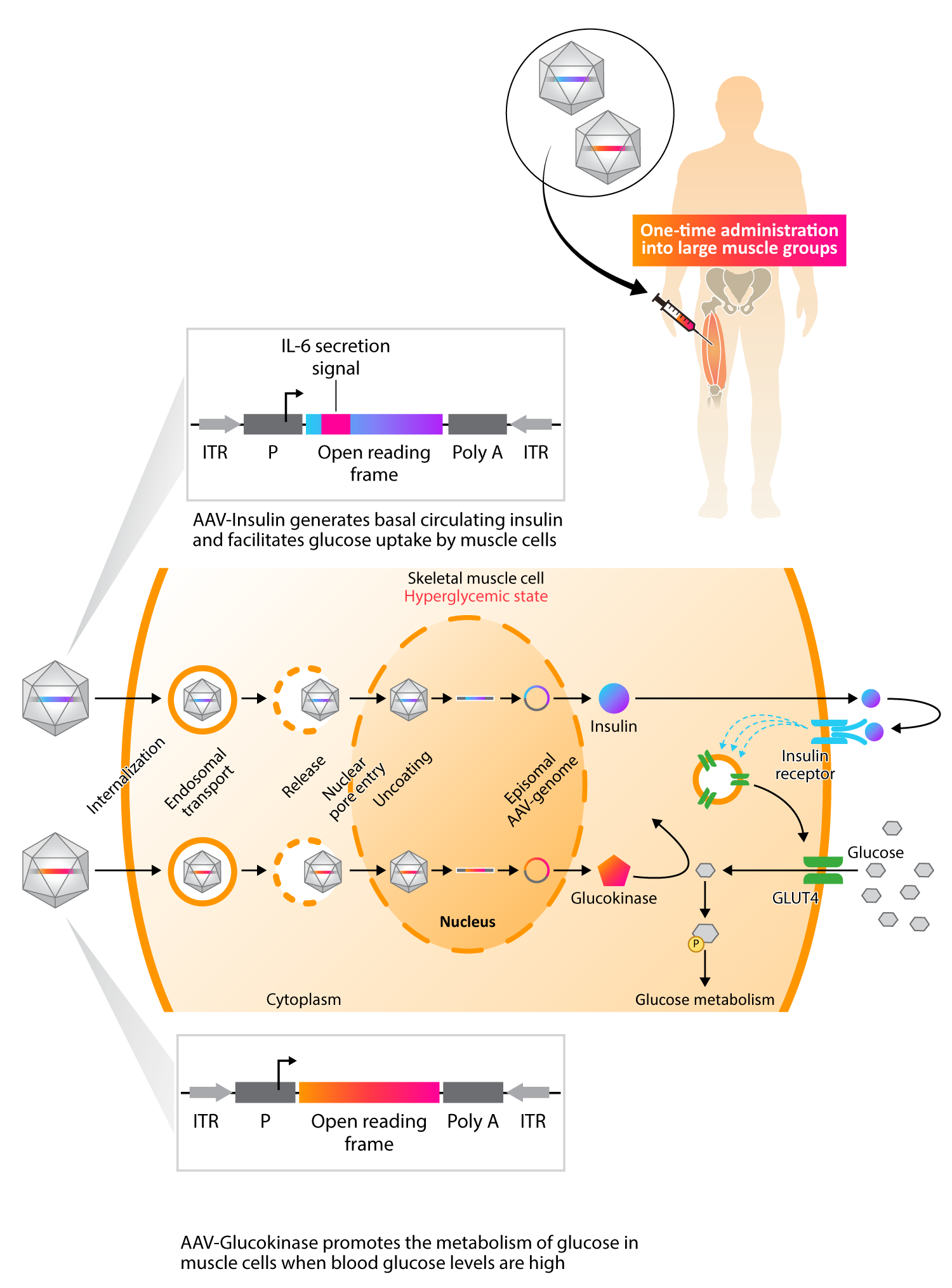
Approach to Treating Diabetes with Gene Therapy
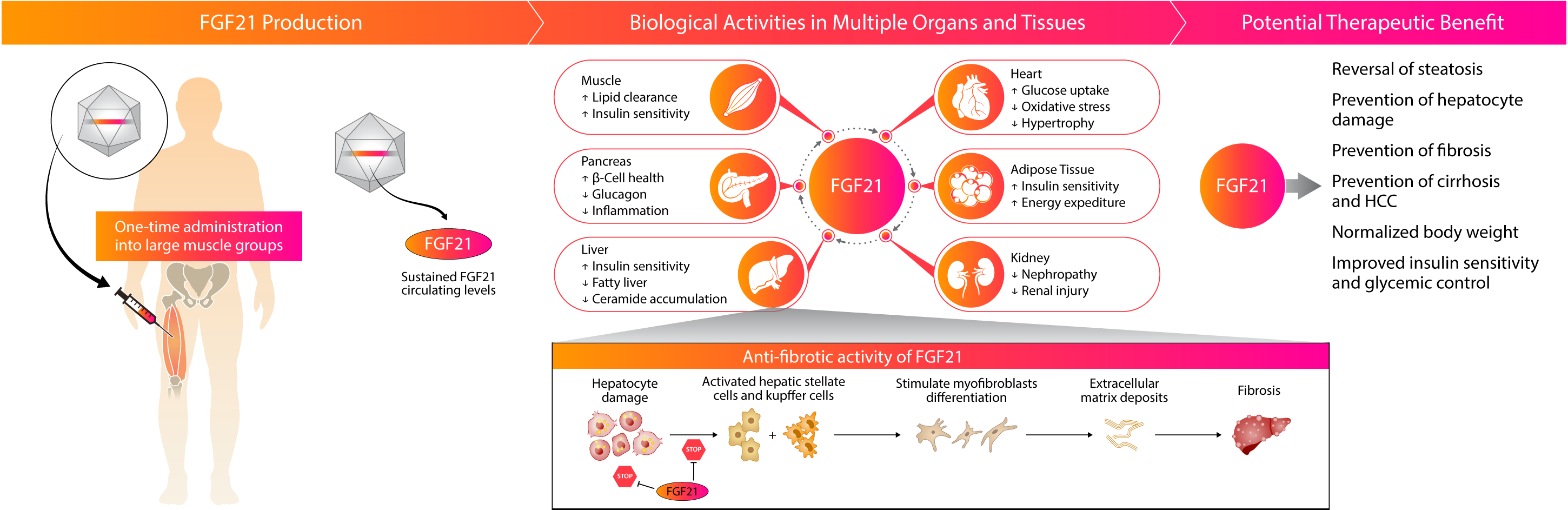
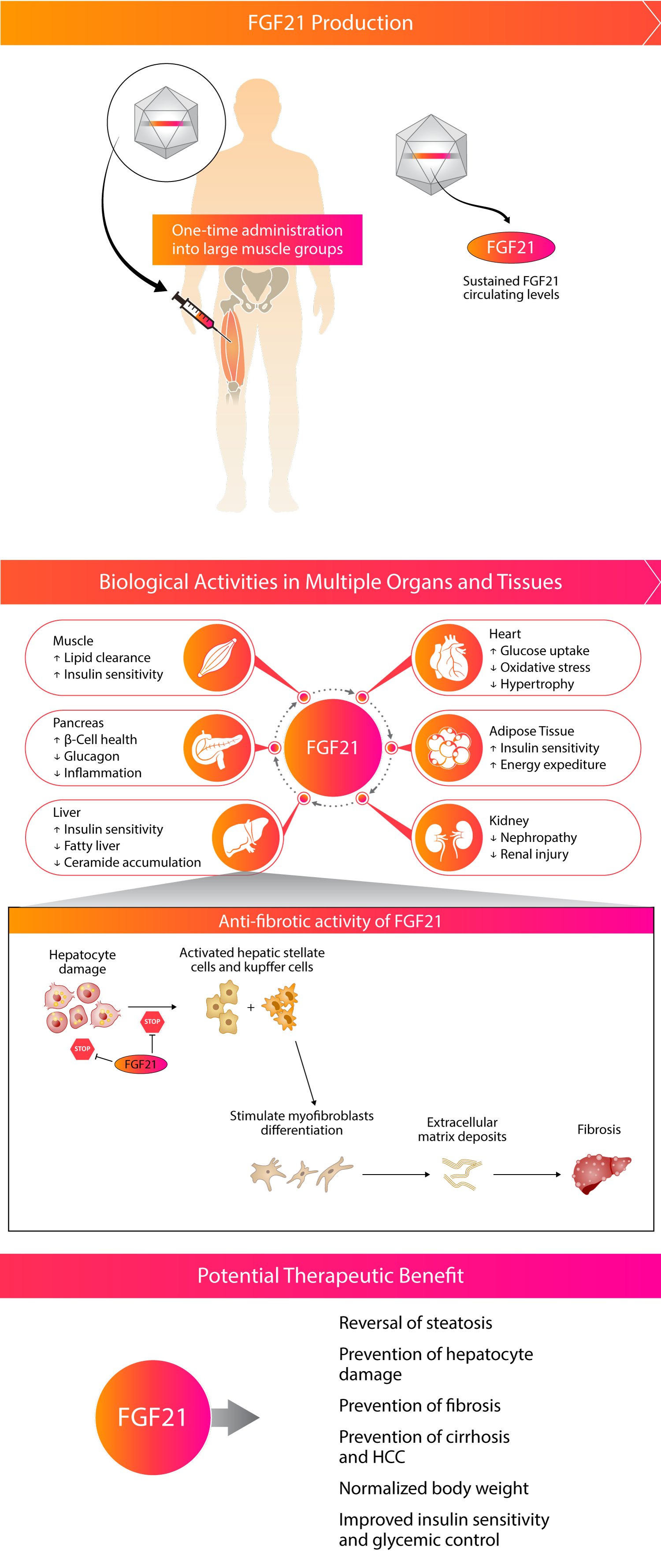
Kriya’s KRIYA-839, an adeno-associated virus (AAV)-based gene therapy, is a groundbreaking approach to restoring insulin production and enhancing glucose metabolism in Type 1 Diabetes (T1D). This approach involves intramuscular delivery of AAV vectors expressing two genes: insulin and glucokinase. These genes are injected into selected large muscle groups, enabling skeletal muscle tissue to secrete insulin and improve glucose metabolism.
Skeletal muscle plays a key role in regulating blood glucose in response to insulin. In healthy individuals, skeletal muscle is responsible for approximately 80% of glucose metabolism and is therefore the primary tissue for glucose disposal. Insufficient production of insulin in T1D prevents skeletal muscle from playing this critical role, causing blood glucose levels to remain elevated.
Kriya’s approach, involving AAV-mediated expression of insulin and glucokinase in skeletal muscles, is designed to restore and augment these muscles’ central role in blood glucose regulation, providing the possibility of a functional cure for T1D.
AAV-mediated expression of insulin in skeletal muscles acts locally to enhance glucose uptake and metabolism by driving translocation of insulin-dependent glucose transporter type 4 (GLUT4) to the muscle cell surface. Combined with increased cell surface GLUT4, AAV-mediated expression of glucokinase in skeletal muscles drives concentration-dependent glucose uptake and metabolism, serving as a “glucose sensor” that promotes uptake only when blood glucose is elevated. Existing data indicates that insulin is also secreted systemically at tonic basal levels to help stabilize fasting blood glucose.
As a result, KRIYA-839 has the potential to create a dynamic, “biological closed loop” system that enables endogenous insulin production and also facilitates glucose uptake and metabolism in response to blood glucose levels. This investigational therapy has the potential to restore blood glucose to a normal range and reduce or eliminate the need for insulin injections, which may allow T1D patients to live a life free from the burden of this debilitating and chronic disease.
NASH
Nonalcoholic steatohepatitis (NASH) is a condition in which excess fat deposits in the liver cause inflammation, cell damage, and fibrosis. Approximately 20 percent of people with NASH will progress to advanced liver disease and associated complications, including cirrhosis and cancer.
NASH affects approximately 40 million people in the United States and European Union.
In March 2024, resmetirom, a once-daily oral medication, became the first FDA-approved treatment for NASH. Resmetirom has only been approved for NASH with stage F2 or F3 fibrosis, meaning that there are still no FDA-approved therapies for the treatment of NASH with stage F4 fibrosis.
Kriya is developing KRIYA‑497, a one-time gene therapy for NASH expressing the native FGF21 protein, delivered intramuscularly with the objective of reducing fibrosis, reversing steatosis, and improving the overall metabolic profile of patients with later stages of NASH with liver-predominant pathology (F3 and compensated F4).
Kriya has designed KRIYA‑497 for NASH with the following potential goals in mind:
- Robust anti-fibrotic activity. Expression of native FGF21 protein to drive natural ligand/receptor interactions and enhance biodistribution across multiple organs
- Minimized treatment burden. One-time intramuscularly administered therapy to reduce complexity for patients who are often taking multiple other concomitant therapies
- Continuous expression. Consistent concentration of native FGF21 protein to limit Cmax-mediated gastrointestinal issues

Approach to Treating NASH with Gene Therapy
Kriya’s gene therapy is designed as a one-time, intramuscular AAV gene therapy with the potential to drive endogenous expression of steady levels of the native FGF21 protein. Multiple published clinical and preclinical studies have supported the anti-fibrotic effect of FGF21 in the liver and other organ systems.