Ophthalmology
Browse to: Geographic Atrophy | Thyroid Eye Disease
Geographic Atrophy
Geographic Atrophy, the advanced form of dry age-related macular degeneration (Dry AMD), affects approximately 2 million people in the United States and European Union. Illustrating the profound societal impact of this disease, approximately 20 percent of cases of legal blindness in North America are caused by Geographic Atrophy.
Patients with Geographic Atrophy suffer from chronic deterioration of cells in the retina, an inner layer of the eye critical for sight, resulting in progressive and irreversible vision loss. Geographic Atrophy can cause blind spots that impair patients’ ability to recognize faces, read, and drive, severely limiting their independence and quality of life.
Existing treatments can be burdensome to patients because they require a regimen of monthly or bimonthly intravitreal injections, administered in-office.
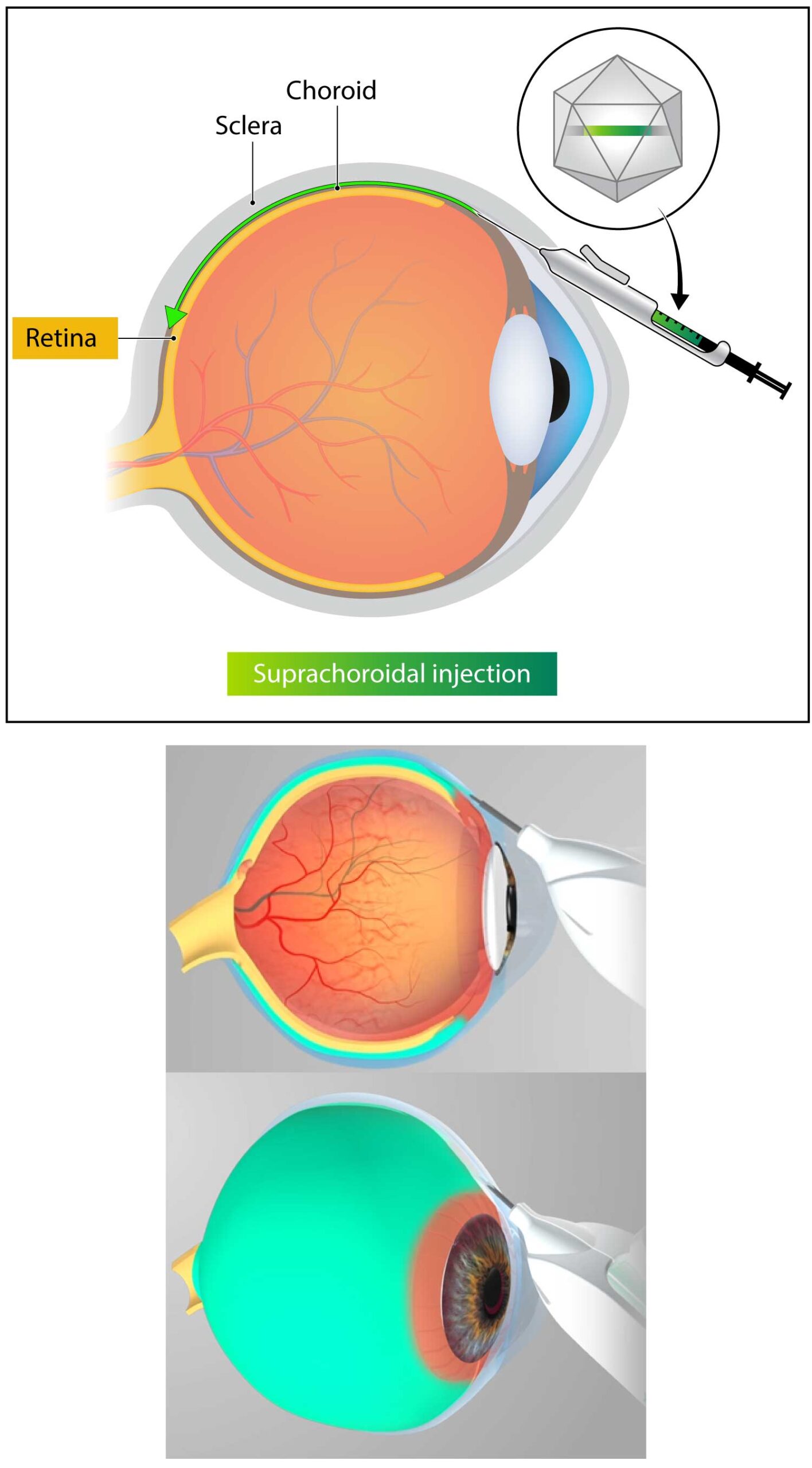
Kriya is developing KRIYA‑825, a one-time gene therapy for Geographic Atrophy that expresses a fusion protein inhibiting complement C3 and C5, with the objective of slowing Geographic Atrophy lesion growth and vision loss. KRIYA-825 will be administered in-office as a one-time, suprachoroidal injection.
Kriya has designed KRIYA‑825 for Geographic Atrophy with the following potential goals in mind:
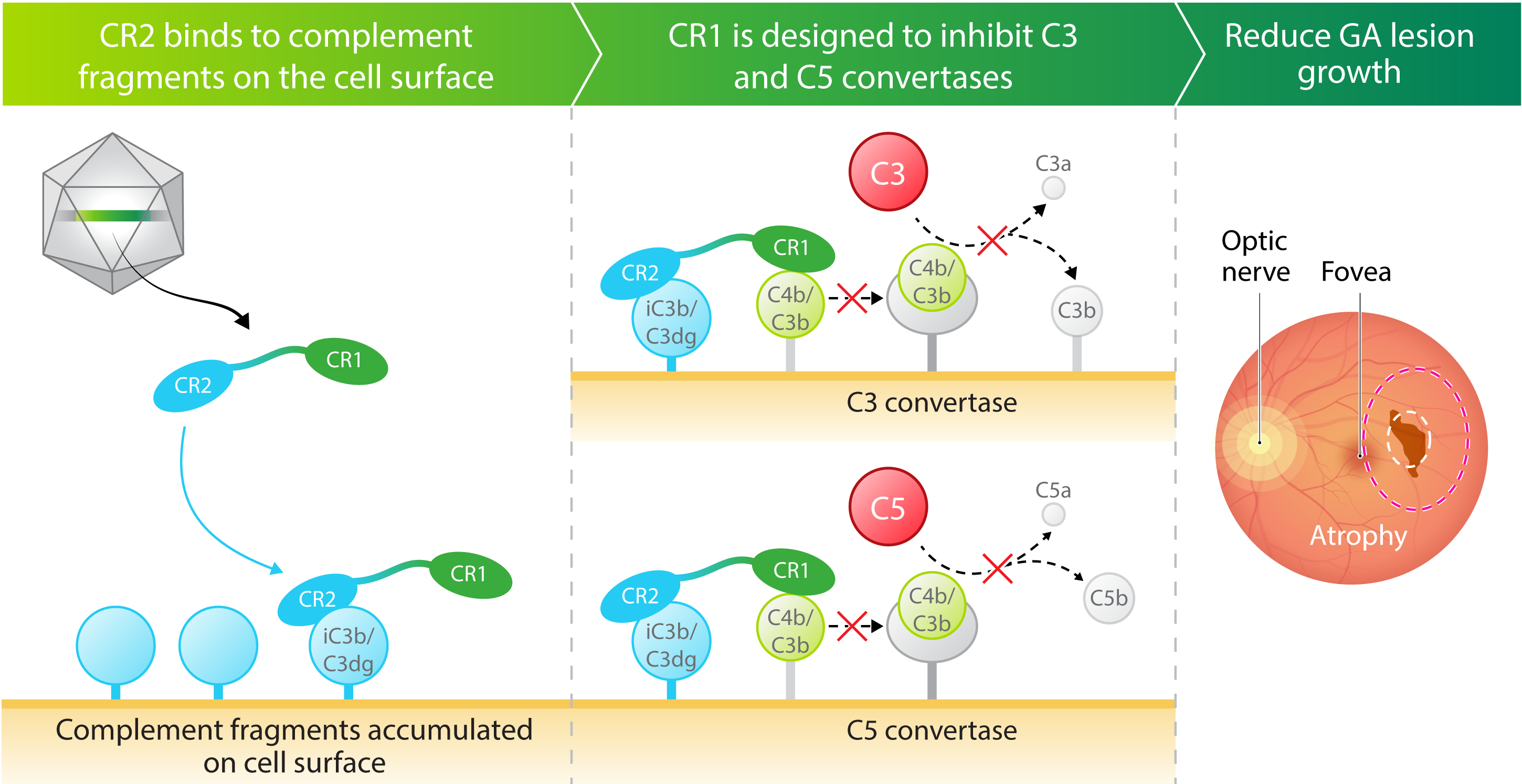
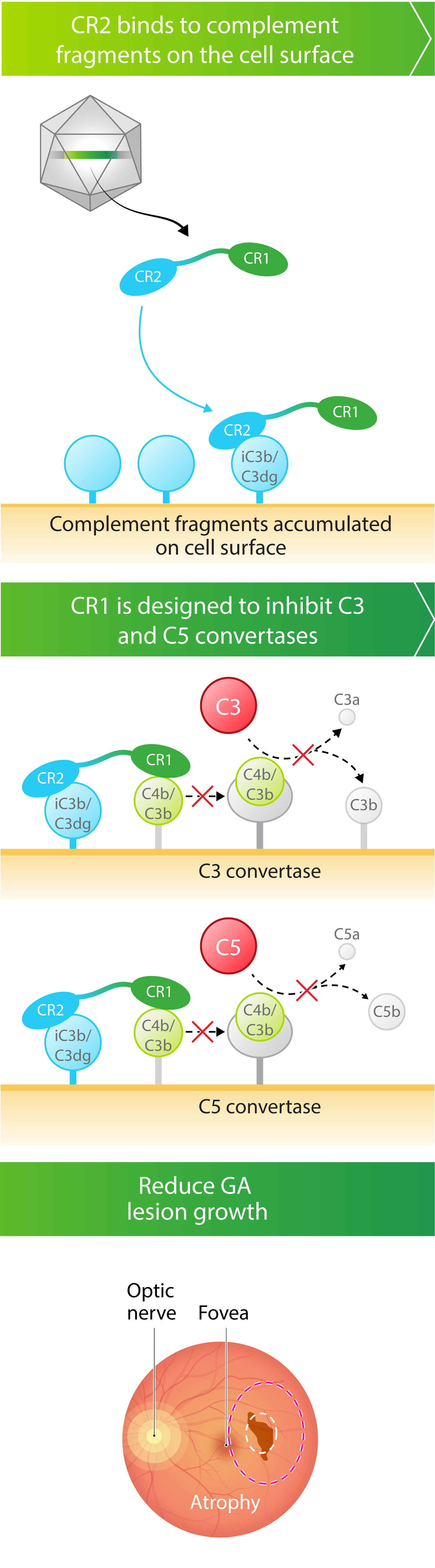
- Robust complement inhibition. A novel complement receptor 2 – complement receptor 1 (CR2‑CR1) fusion protein-where the CR1 domain is designed to block the function of both C3 and C5, while the CR2 domain is designed to bind to cell surfaces where complement fragments deposit and cause damage
- Multi-year durability. Adeno-associated virus (AAV)-mediated continuous expression of CR2‑CR1 fusion protein following a one-time injection to eliminate the need for frequent intravitreal injections as required by currently available therapies
- Targeted delivery. One-time suprachoroidal injection with the goal of achieving transduction of retinal cells while minimizing inflammation and overall patient burden

Approach to Treating Geographic Atrophy with Gene Therapy
Kriya’s AAV gene therapy encodes a fusion protein that combines critical domains of complement receptor 2 (CR2) and complement receptor 1 (CR1). CR1 is designed to inhibit the C3 and C5 pathways in the complement cascade, while CR2 prolongs and enhances the inhibitory effect of CR1 by binding the protein to complement fragments deposited on the cell surface, where they accumulate and cause damage. Taken together, the AAV-expressed CR2‑CR1 fusion protein may provide multiyear therapeutic benefit following a one-time injection.
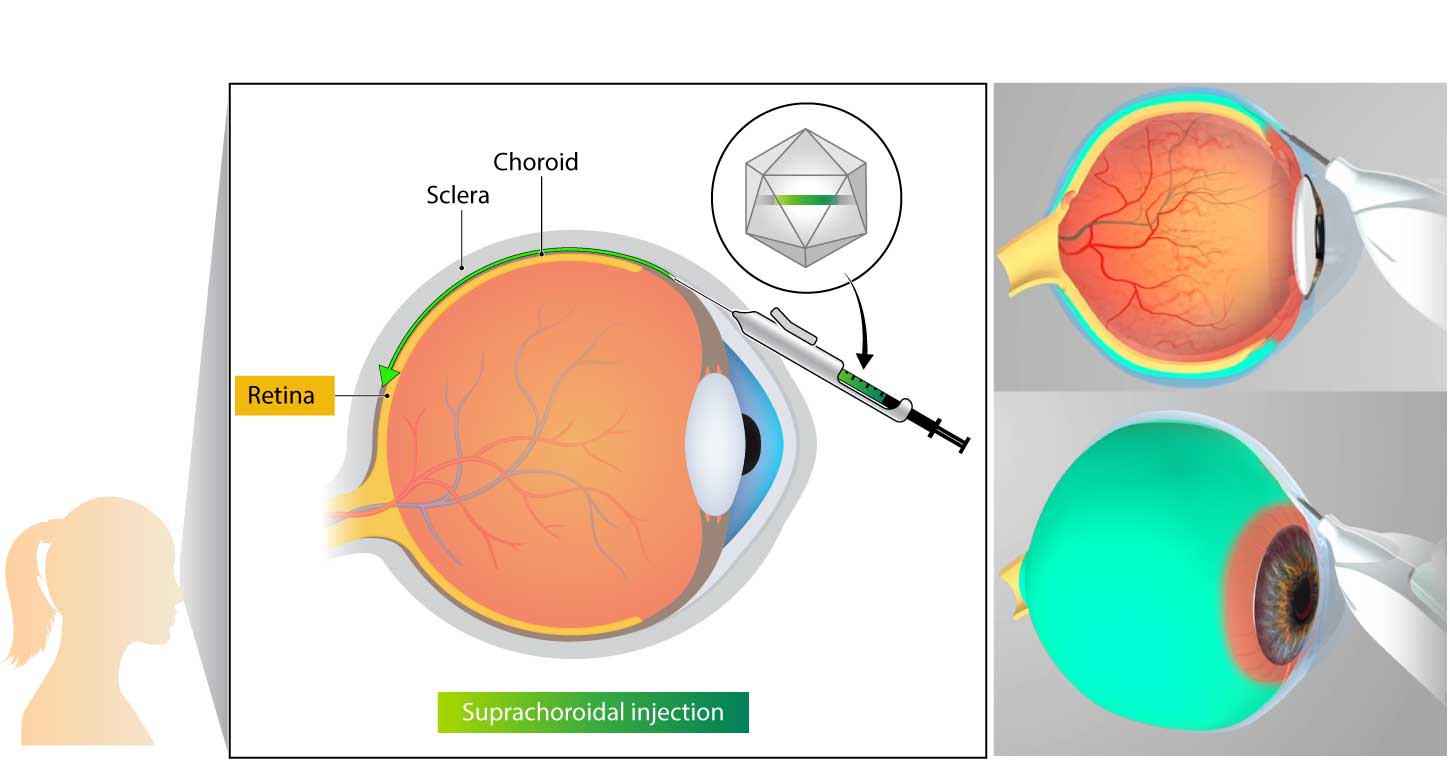
Kriya uses an investigational suprachoroidal delivery device that enables non-surgical targeted delivery of its gene therapy to the retina and choroid via the suprachoroidal space. The delivery device utilizes a geometrically-optimized, non-sharp tissue separator to open a path into the suprachoroidal space, enabling a tangential injection that leads to rapid, broad distribution of the drug into the retina.
Thyroid Eye Disease
Thyroid Eye Disease is a debilitating autoimmune disease that causes inflammation and enlargement of the muscles and fatty tissue behind the eye. This results in proptosis (bulging of the eyes) and diplopia (double vision), which can significantly impact patients’ quality of life.
Approximately 1 million people in the United States and European Union have Thyroid Eye Disease.
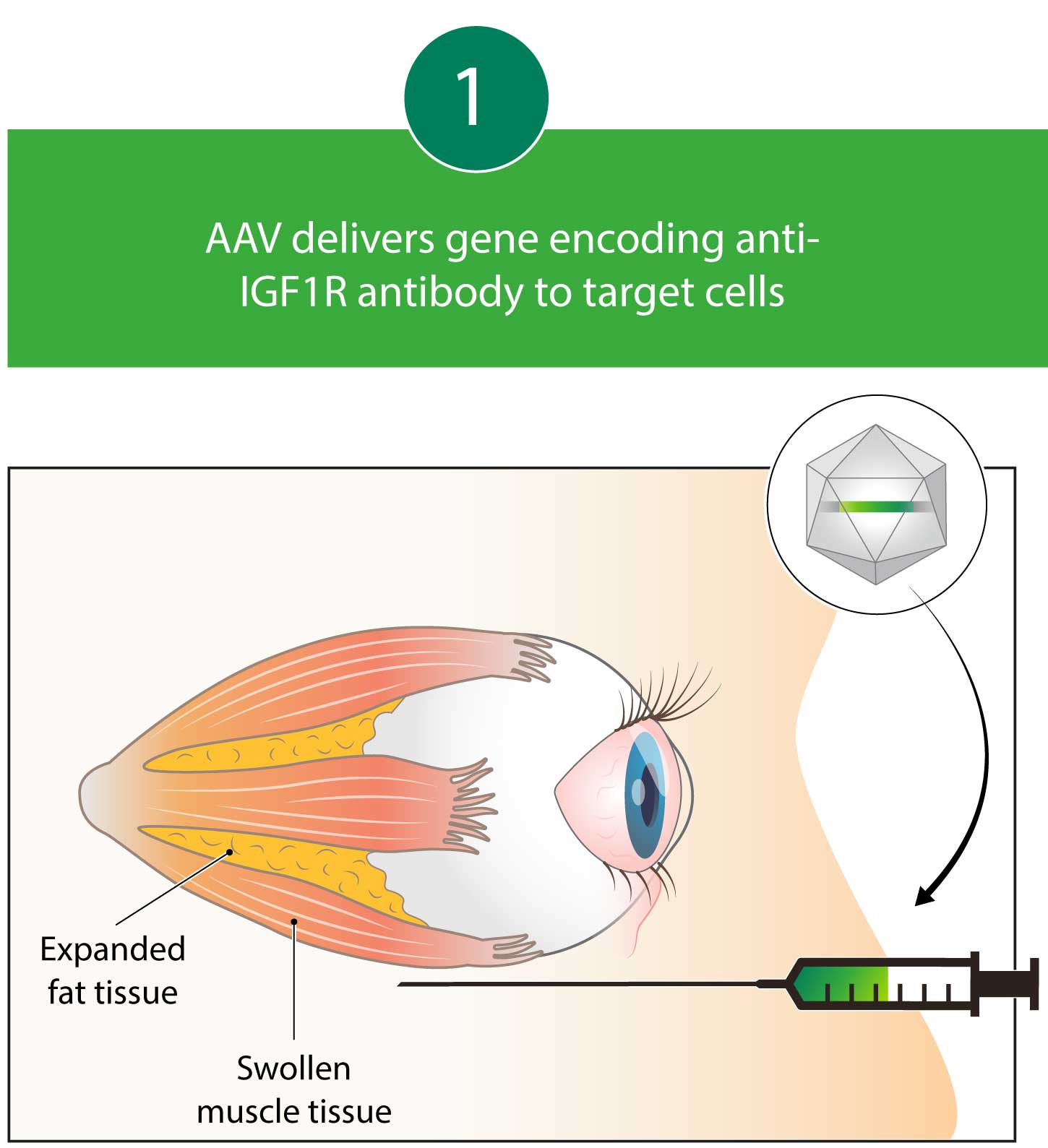
Kriya is developing KRIYA‑586, a one-time gene therapy for Thyroid Eye Disease that expresses an antibody designed to block receptors for Insulin-like Growth Factor 1 (IGF1), with the objective of achieving sustained improvements in proptosis, diplopia, and other symptoms. KRIYA-586 will be administered in-office as a one-time, peribulbar injection into the fat around the eye.
FDA approval of teprotumumab, an IGF1 receptor antagonist and the only approved therapy for Thyroid Eye Disease in the United States, has established IGF1 receptor blockade as a clinically-validated mechanism of action in this disease.
Kriya has designed KRIYA‑586 for Thyroid Eye Disease with the following potential goals in mind:
- Minimized treatment burden. One-time, in-office peribulbar injection to eliminate the burdensome requirement for multiple infusions or injections
- Focal delivery. Localized antibody expression in extraocular muscles and fat tissue to limit the potential for side effects that can be observed with systemically administered therapies
- Long-term durability. AAV-mediated antibody expression to deliver sustained improvements in proptosis and diplopia as well as other key manifestations of Thyroid Eye Disease

Approach to Treating Thyroid Eye Disease with Gene Therapy
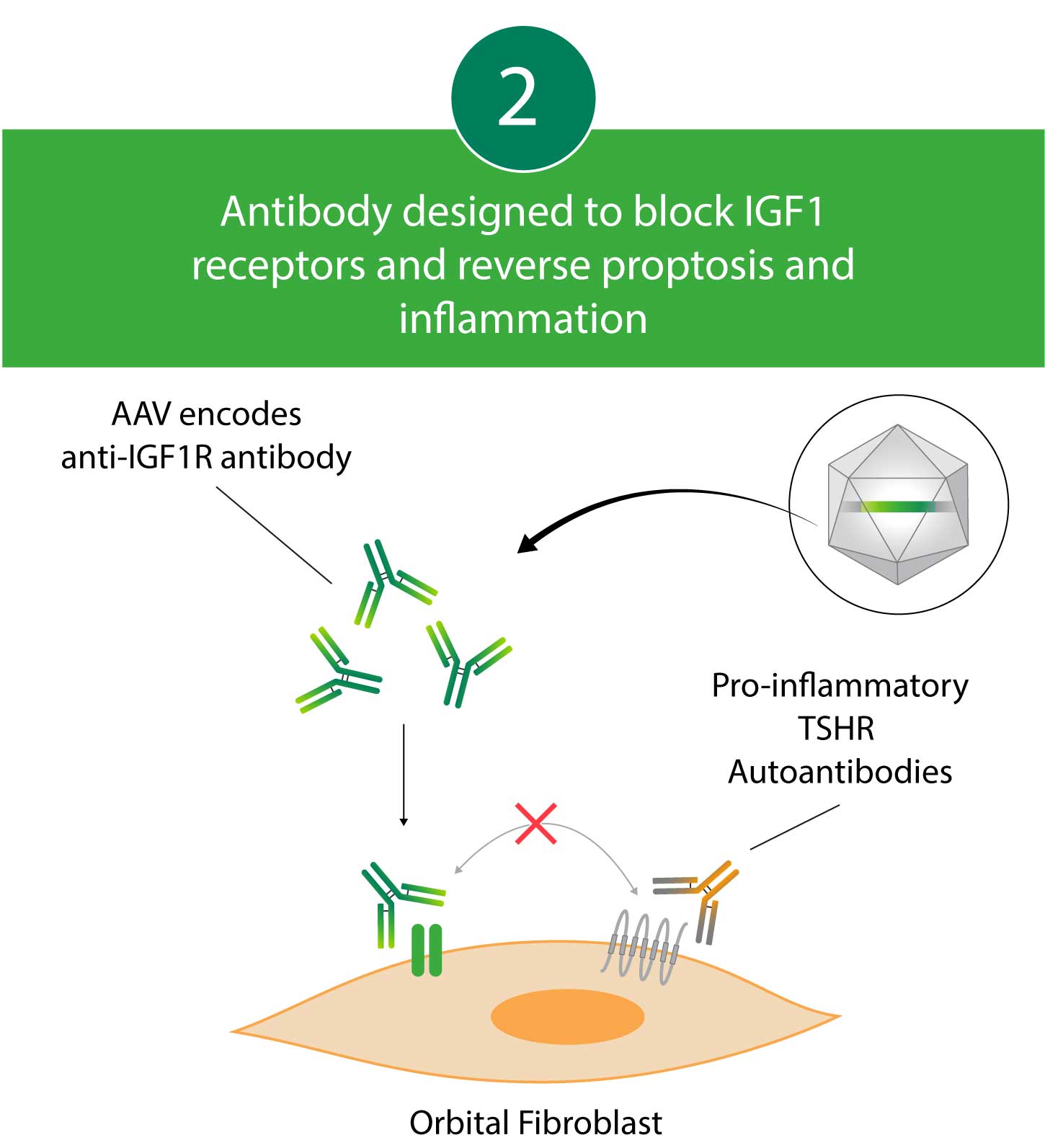
Kriya’s AAV gene therapy encodes an antibody that blocks Insulin-like Growth Factor 1 (IGF1) receptors. By blocking IGF1 receptors, this antibody decreases pro-inflammatory Thyrotropin Receptor (TSHR) autoantibody signaling, which may lead to reductions in proptosis and diplopia. Kriya’s gene therapy is designed for administration as a one-time, focal, peribulbar injection into fatty tissue around the eye, with a goal of achieving local antibody expression and minimizing systemic exposure.